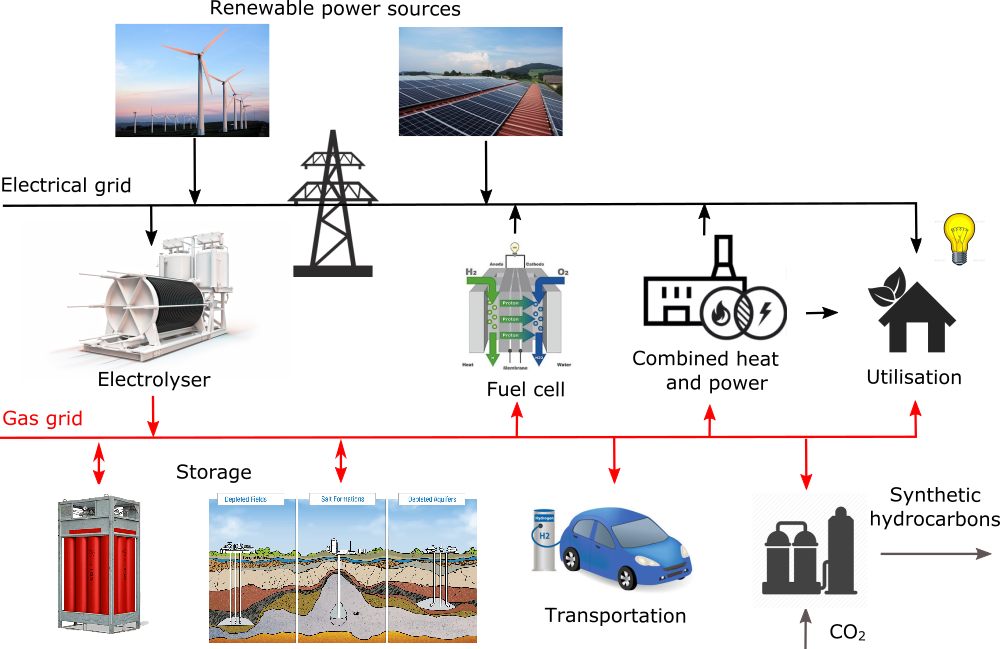
In recent decades, renewable energy sources like wind power and photovoltaics have become a mature wide-spread technology. The increase of their share in power mix is limited by their intermittent operation and by the lack of suitable energy storage technology. Electrolysis of water represents a crucial step in so-called Power-to-gas (PtG) conversion, where excessive renewable power is used for water splitting to generate hydrogen. The hydrogen can be handled directly as a zero-emission fuel, or can serve as a feedstock for production of synthetic fuels.
The advantage of such concept compared to other energy storage technologies lie in great possibilities of scaling and decentralisation, so that capacity of renewable power integrated into power system could be practically unlimited. Hydrogen distribution network could also interconnect many sectors like industry, transportation and residential.
Development of separator membranes for alkaline water electrolysis
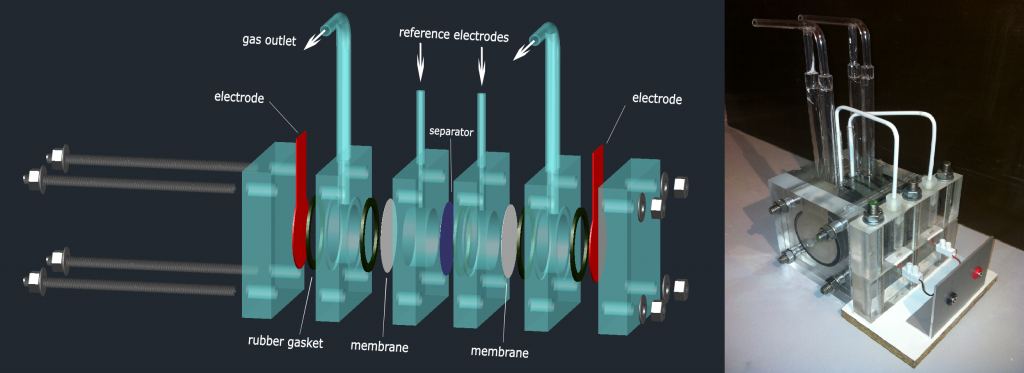
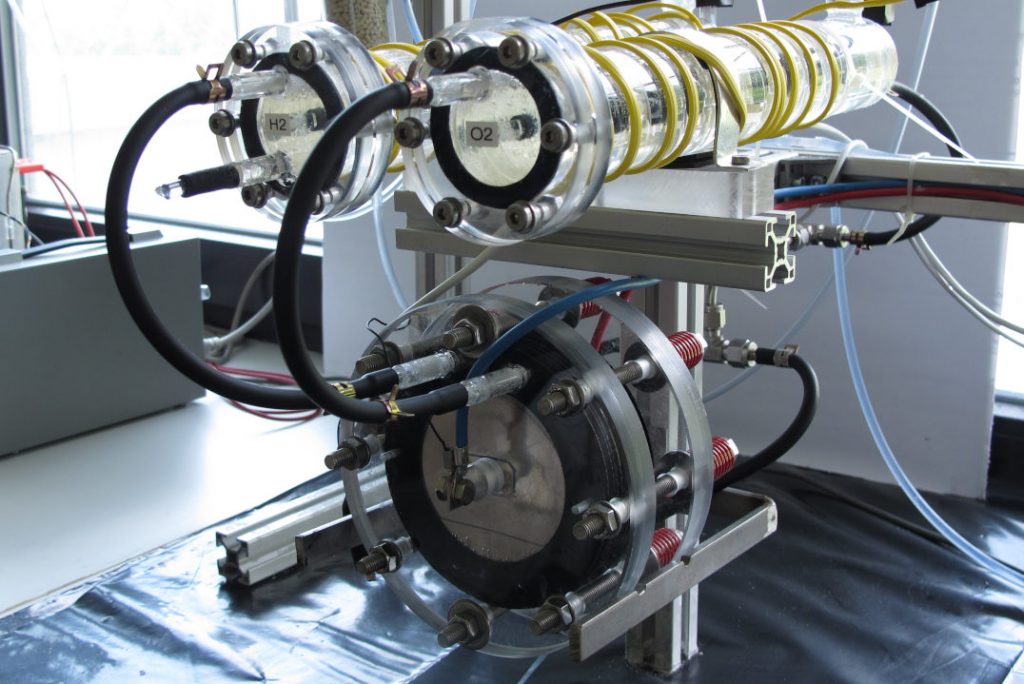
Our research is dedicated to the development of alkaline water electrolyser suited for intermittent operational conditions. This includes development of separator membranes, electrolysis cell stack and gas purity detector
Objectives
- High wettability by aqueous electrolytes.
- High electrolytic conductivity.
- Low permeability of dissolved H2 and O2.
- Low hydraulic conductivity.
- Durability under electrolysis conditions.
- Based on inexpensive materials
Preparation of hydrophilic membranes is based on activation of microporous polymeric substrates by non-equilibrium plasma (low-pressure discharge) followed by graft polymerization of a suitable monomer (e.g. acrylic acid) on plasma-activated membranes.


Characterization of separator membranes
- Determination of electrolytic conductivity in a four-contact electrolysis cell.
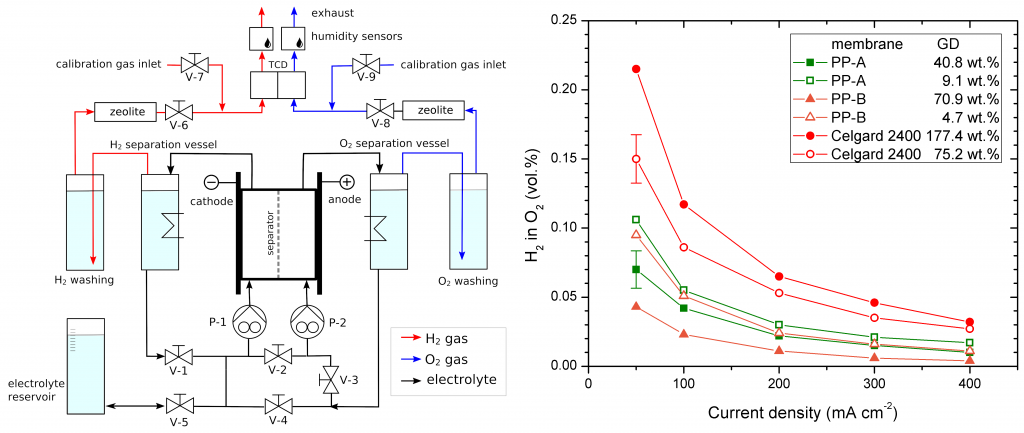
- Determination of gas permeability under electrolysis conditions from mutual cross-contamination of the produced hydrogen and oxygen.
- Long-term performance of separators under electrolysis conditions.
- Gravimetric analysis.
- Structural analysis by Scanning Electron Microscopy (SEM).
- Chemical composition by FTIR.
- Wettability by Critical Wetting Surface Tension (CWST) test.
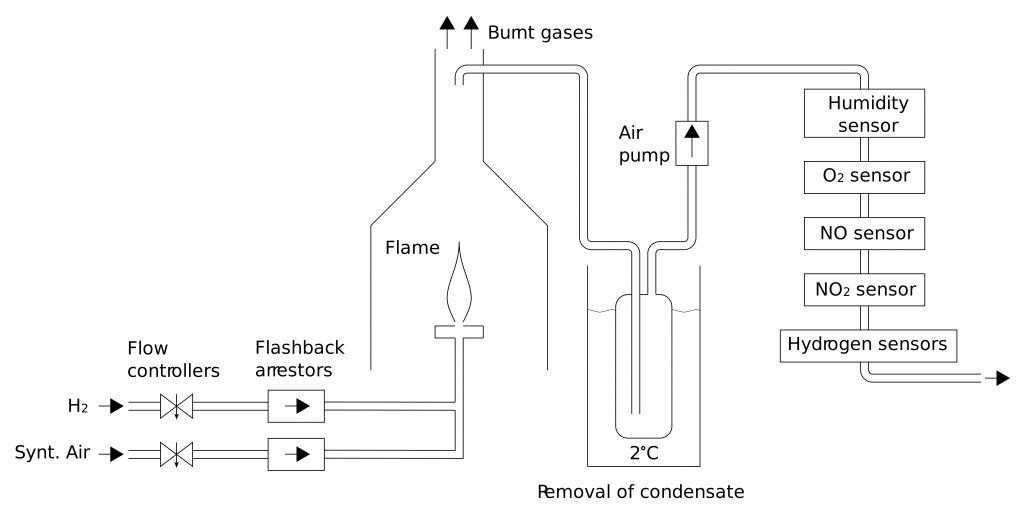
Optimization of hydrogen flame burners
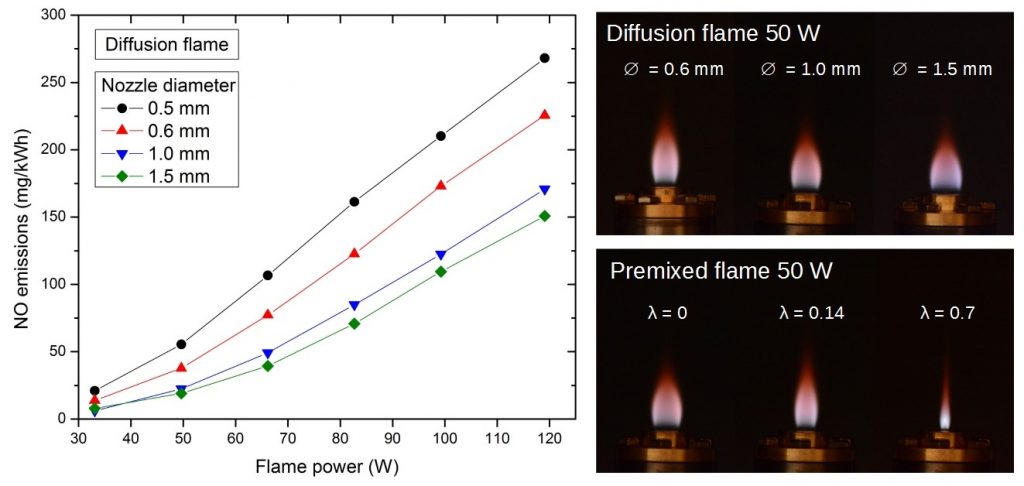
Although hydrogen is a clean fuel, its combustion at elevated temperature initiates (similarly to combustion of other fuels) reactions of atmospheric N2 and O2, resulting in formation of nitrogen oxides. NO and NO2 have adverse health effects, therefore their formation should be minimized. This is especially important for indoor gas appliances like stoves. Moreover, combustion characteristics of hydrogen (e.g. heating value, air requirement, burning velocity) considerably differ from those of common hydrocarbon fuels like methane or propane. Burners combusting hydrogen therefore require different design.
Objectives
- Study of NOx formation in hydrogen flames in air.
- Study of stability of premixed hydrogen flames in respect to flashback and flame lift.
- Design of variable-power burners with high turn-down ratio and low emissions of NOx.

Contacts:
Michal Stano, PhD.
F2 – 84
Email: stano(at)fmph.uniba.sk
Phone Nr.: +421 2 602 95 240
Ľubomír Staňo, PhD.
F2 – 74
Email: lubomir.stano(at)fmph.uniba.sk
Phone Nr.: +421 2 602 95 581